Development of New Candidate Agent for Lethal and Severe Cutaneous Drug Reaction
Oct 24 2025
Discovery of Inhibitors that Suppress Disease-Specific Keratinocyte Death and Demonstration of Their Efficacy in Preclinical Studies
A collaborative research group led by Haruna Kimura (graduate student), Dr. Akito Hasegawa (Assistant Professor), and Prof. Riichiro Abe from the Division of Dermatology, Niigata University Graduate School of Medical and Dental Sciences, together with Prof. Takemasa Ozawa from the Department of Chemistry, School of Science, The University of Tokyo, and Dr. Yoichi Ogawa (Lecturer) from the Department of Dermatology, Faculty of Medicine, University of Yamanashi, has developed a novel therapeutic candidate that may improve the prognosis of severe cutaneous adverse reactions such as Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN).
SJS/TEN are severe diseases with a high mortality rate of approximately 30%. The research group previously revealed that a type of programmed cell death known as necroptosis, mediated through formyl peptide receptor 1 (FPR1), occurs in lesional skin of SJS/TEN patients. In this study, the team developed an inhibitor that suppresses necroptosis, demonstrating that it effectively reduced cell death in SJS/TEN model cells and prevented disease onset in model mice. The results of this study were published in Nature Communications on September 30, 2025.
SJS/TEN are triggered by drug administration and characterized by erosion of the skin and mucous membranes. According to Japanese clinical guidelines, systemic corticosteroids are the first-line treatment, and in refractory cases, intravenous immunoglobulin or plasma exchange therapy is used. However, about 30% of patients still suffer fatal outcomes, highlighting the urgent need for novel and more effective therapeutic options.
The research group previously discovered that necroptosis occurs in keratinocytes within SJS/TEN lesions and that this process is induced through stimulation of FPR1, a receptor expressed on epidermal cells. Therefore, in this study, the team developed a screening system to identify compounds with strong FPR1 inhibitory activity and used SJS/TEN model cells to demonstrate the potential efficacy of FPR1 inhibitors as novel therapeutic agents.
The team screened for potent FPR1 inhibitors using the compound library of the Drug Discovery Initiative, The University of Tokyo. FPR1 belongs to a receptor family known as G protein–coupled receptors (GPCRs), which function through signaling pathways mediated by both G proteins and β-arrestins. Prof. Ozawa’s group had previously developed G-protein and β-arrestin assays capable of detecting each signaling pathway independently. Using these assays, the researchers screened the Tokyo University compound library and selected two candidate compounds showing high FPR1 inhibitory activity. In addition, five other compounds previously reported to inhibit FPR1 were included as reference candidates.

Dr Haruna Kimura, the first author of the paper.
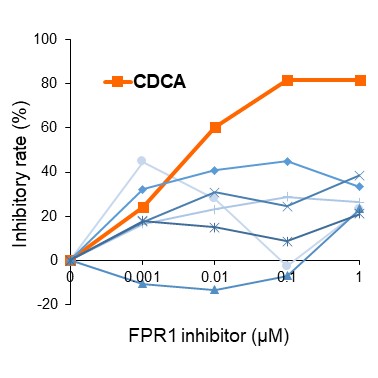
Figure 1: Validation of FPR1 inhibitor candidates using SJS/TEN model cell
The FPR1-inhibitory activity of the candidate compounds was evaluated using an in vitro SJS/TEN assay. Cell death inhibition was assessed by live/dead staining following treatment with various concentrations of each compound. Among the seven candidates, CDCA exhibited a specific and potent inhibitory effect on cell death, even at low concentrations.
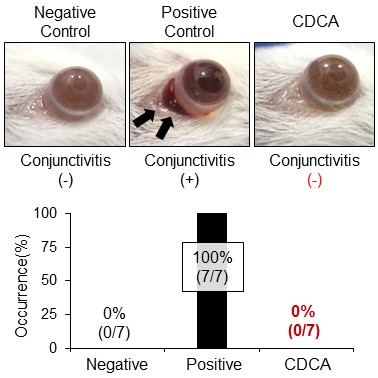
Figure 2: CDCA completely suppressed conjunctivitis in SJS/TEN model mice
To confirm the in vivo effectiveness of CDCA, the compound was administered to SJS/TEN mouse model that closely mimics the pathological features observed in human patients. Mice in the positive control group exhibited marked eyelid conjunctivitis (n = 7), whereas the CDCA-treated group showed no signs of conjunctivitis (n = 7)
Publication Details
Journal: Nature communications
Title:Inhibition of formyl peptide receptor-1-mediated cell death as a therapy for lethal cutaneous drug reactions in preclinical models
Authors:Haruna Kimura, Akito Hasegawa*, Tomoki Nishiguchi, Hong Ha Nguyen, Masatoshi Eguchi, Manao Kinoshita, Youichi Ogawa, Takeaki Ozawa*, Riichiro Abe*
*:corresponding author
Doi: 10.1038/s41467-025-63744-0
News release
The article was released in EurekAlert, the online publication of the American Association for the Advancement of Science.
More News
-
 Feb 13 2026 Research results
Feb 13 2026 Research resultsEvaluation of plasma p-tau217 biomarkers in detecting amyloid pathology and predicting cognitive outcomes: Observations from Japanese Alzheimer's disease neuroimaging initiative cohort
-
 Feb 12 2026 Research results
Feb 12 2026 Research resultsFrom surface to depth: 3D imaging traces vascular amyloid spread in the human brain
-
 Jan 28 2026 Research results
Jan 28 2026 Research resultsTreatment initiation is possible with a positive liquid biopsy in primary central nervous lymphoma patients with difficult-to-access lesions
