Assessment of Liver Lobe-specific Hydrodynamic Gene Delivery to Baboons: A Preclinical Trial for Hemophilia Gene Therapy
Jun 06 2023
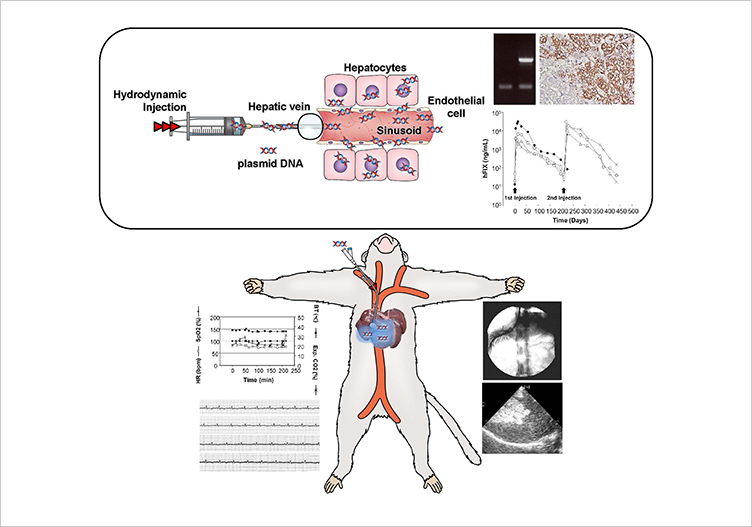
Gene therapy is attracting attention as a treatment method for intractable diseases. Therefore, establishing safer and more therapeutically effective gene therapy procedures for disease treatment is essential. A research group led by Professor Kenya Kamimura (Department of General Medicine, Niigata University School of Medicine/Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University) and Professor Dexi Liu (Department of Pharmaceutical and Biomedical Sciences, College of Pharmacy, University of Georgia) has demonstrated the efficacy and safety of a hydrodynamic gene delivery procedure1 in large animals. In this study, they have shown the clinical applicability of the procedure and conducted the preclinical study for hemophilia gene therapy using baboons. They have demonstrated that lobe-specific hydrodynamic gene delivery to liver of the baboons can be safely applied and showed therapeutic effect for treating hemophilia2.
Research results
- Gene therapy is attracting attention as a treatment method for intractable diseases. Therefore, establishing safer and more therapeutically effective gene therapy procedures for disease treatment is essential.
- The liver lobe-specific hydrodynamic delivery procedure developed by this research group was applied to primates (baboons) for the first time.
- The results demonstrated liver lobe-specific gene delivery, therapeutic levels of human factor IX gene expression lasting for 200 days after the delivery of a plasmid that expresses a therapeutic gene for human hemophilia3, and the efficacy of repeated hydrodynamic gene delivery into the same liver lobes. Other than a transient increase in blood concentration of liver enzymes right after the injection, no significant adverse events were observed in animals during the study period.
- We confirmed that no plasmid was introduced into organs other than the livers of the baboons treated with gene therapy.
[Glossary]
-
Hydrodynamic gene transfer method
A method that employs a physical force generated by the combined effect of injection speed and volume of gene-containing solution that permeabilizes cell membrane and accomplishes intracellular gene transfer. The authors have extensively studied the procedure to apply for gene therapy for diseases and for animal model development. (Kamimura K, et al. Mol Ther, 2009; Kamimura K, et al. Mol Ther, 2010; Yokoo T & Kamimura K, et al. Gene Ther, 2013; Kamimura K, et al. Mol Ther Nucleic Acids, 2013; Abe H & Kamimura K, et al. Mol Ther Nucleic Acids, 2016; Kobayashi Y & Kamimura K, et al. Mol Ther Nucleic Acids, 2016; Ogawa K & Kamimura K, et al. Mol Ther Nucleic Acids, 2017; Shibata O & Kamimura K, et al. Mol Ther Nucleic Acids, 2022) -
Hemophilia
A disease that impairs the function of blood clotting to stop bleeding mostly due to a genetic disorder resulted in the low levels of clotting factor proteins. -
Plasmid
Circular double-stranded DNA that exists outside the nucleus in bacteria and replicates independently of chromosomal DNA. It is also used to transport the gene to cells and express the protein.
Publication Details
Journal: Molecular Therapy-Nucleic Acids
Title: Liver Lobe-specific Hydrodynamic Gene Delivery to Baboons: A Preclinical Trial for Hemophilia Gene Therapy
Authors: Kenya Kamimura, Tsutomu Kanefuji, Takeshi Suda, Takeshi Yokoo, Guisheng Zhang, Yutaka Aoyagi, and Dexi Liu
doi: 10.1016/j.omtn.2023.05.018
News release
The article was released in EurekAlert, the online publication of the American Association for the Advancement of Science.
More News
-
 Jul 07 2025 Research results
Jul 07 2025 Research resultsFertilizable rat sperm produced in a mouse body by blastocyst complementation
-
 Jun 26 2025 Research results
Jun 26 2025 Research resultsEstimating Microbial Biomass from Air-Dried Soils: A Safer, Scalable Approach ーRevolutionary Technique Estimates Soil Microbial Biomass Using Water-Extractable Organic Matterー
-
 Jun 03 2025 Research results
Jun 03 2025 Research resultsAssociation of rare APOE missense variants with Alzheimer's disease in the Japanese population
