Identification of a protein that inhibits ubiquitination and aggregation of α-synuclein, a causative factor of Parkinson's disease
Sep 10 2019
Parkinson's disease is a progressive neurodegenerative disorder characterized by loss of dopaminergic neurons in the substantia nigra and striatum of brains. α-synuclein is the causative protein of Parkinson's disease. Ubiquitinated α-synuclein aggregates in nerve cells play a central role in the development of disease. A research team at Niigata University has discovered that G3BP1 protein inhibits ubiquitination and aggregation of α-synuclein. This study suggested that the G3BP1 plays a protective role in the development of Parkinson's disease by reducing α-synuclein ubiquitination and aggregation. Therefore, G3BP1 is a promising drug target for the treatment of Parkinson's disease.
Publication Details
Title: G3BP1 inhibits ubiquitinated protein aggregations induced by p62 and USP10
Journal: Scientific Reports
Authors: Sergei Anisimov, Masahiko Takahashi, Taichi Kakihana,Yoshinori Katsuragi, Hiroki Kitaura, Lu Zhang, Akiyoshi Kakita, Masahiro Fujii
More News
-
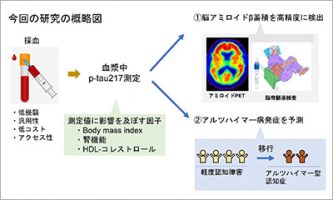 Feb 13 2026 Research results
Feb 13 2026 Research resultsEvaluation of plasma p-tau217 biomarkers in detecting amyloid pathology and predicting cognitive outcomes: Observations from Japanese Alzheimer's disease neuroimaging initiative cohort
-
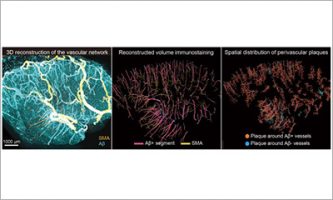 Feb 12 2026 Research results
Feb 12 2026 Research resultsFrom surface to depth: 3D imaging traces vascular amyloid spread in the human brain
-
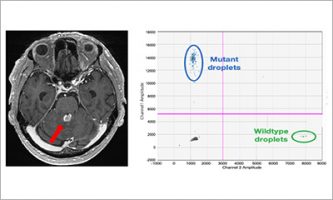 Jan 28 2026 Research results
Jan 28 2026 Research resultsTreatment initiation is possible with a positive liquid biopsy in primary central nervous lymphoma patients with difficult-to-access lesions
